Cefotaxime SITIMIXIE Sahar Pharma

Buy Cefotaxime ( Generic Claforan ) Online Cefotaxime Injection buypharma.md
Find patient medical information for cefotaxime injection on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.

Cefotaxime Atb 1g IV Pharmatech Company for Drugs and Medical Supply
The present invention discloses a method for synthesizing cefotaxime sodium, and said method includes the following steps: placing 7-amino cephaloalkyl acid (7-ACA) and benzothiazol active ester (MEAM) in organic solvent, using triethylamine as condensation agent, under the catalysis of phase transfer catalyst making them produce condensation reaction to obtain cefotaxime acid, and making the.
Cefotaxime Sodium Injection IP (Omnatax 1 gm) by Healthy Inc., cefotaxime sodium injection
Cefotaxime is a medication used to manage and treat cervicitis/urethritis and pneumonia. Cefotaxime is a beta-lactam antibiotic classified as a third-generation cephalosporin. Its broad-spectrum antibacterial activity is useful in treating the susceptible strains of bacteria affecting the lower respiratory tract, genito-urinary tract, central nervous system, intra-abdominal infections, bone.

Jual obatinfeksicefotaxime1gram1box10vials di Lapak abil herbals Bukalapak
US patent 4,152,432 discloses the process for the preparation of cefotaxime sodium of formula (I), which involves treating the cefotaxime acid in aqueous solvent such as methanol, ethanol or acetone in the presence of base and sodium ions to give cefotaxime sodium. US patent 5,831,086 describes the process for the preparation of sodium cefotaxime.
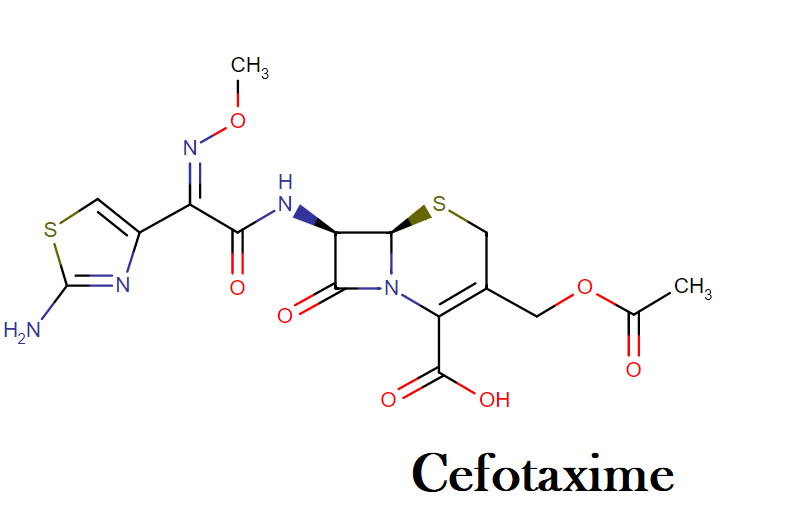
Cefotaxime Drug class, mechanism of action, uses, dosage, side effects and interactions
PATIENT & CAREGIVER EDUCATION Cefotaxime This information from Lexicomp explains what you need to know about this medication, including what it's used for, how to take it, its side effects, and
cefotaxime for injection_Tenas
Production of the sodium salt of cefotaxime 6 g of sodium acetate trihydrate are dissolved in 20 ml of water. 40 ml of acetone and 20 g of cefotaxime in free acid form are added to the stirred solution. The mixture is cooled to 0°. A solution is obtained within 2 to 5 minutes.
Cefotaxime, Powder 500mg/Vial, SDV, 10Vials/Tray McGuff Medical Products
The present invention relates to an improved process for the production of 7- [2- (2-aminpthiazol-4-yl)-2-syn-methoxyiminoacetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid (Cefotaxime) and its sodium salt. The synthesis of Cefotaxime includes the reaction of 2- (2-chloroacetamidothiazol-4-yl)-2-syn-methoxyiminoacetyl chloride with 7.

Cefotax Cefotaxime Sodium Injection IP 1gm at Rs 36/vial in Vasai Virar ID 15589945555
A process for the production of 7-[2-(2-amino-4-thiazolyl)-2-syn-methoxyimino-acetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid (Cefotaxime) in aqueous isopropyl alcohol is provided. The synthesis provides the product in greater than 99 % HPLC purity.
cefoTAXime for Injection BP 2 g SteriMax Inc.
CN-111647006-B chemical patent summary. An official website of the United States government. Here is how you know

Cefotaxime 1g Mekophar
Cefotaxime | C16H17N5O7S2 | CID 5742673 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.

سيفوتاكسيم Cefotaxime صحتنا
Patent Expirations for CEFOTAXIME SODIUM ⮫ Send this page by email. Email this page to a colleague

Cefotaxime Side Effects, uses and dosage
Cefotaxime injection is used to treat bacterial infections in many different parts of the body. This medicine is also given before, during, and after certain types of surgery to prevent infections. Cefotaxime injection belongs to the class of medicines known as cephalosporin antibiotics.

CEFOTAXIME 1g Powder for Injection Bernofarm
Crystalline sodium salt of cefotaxime is separated by filtration, washed with acetone and dried overnight in a vacuum drying chamber at 40°. Yield: 36.6 g; Content of the sodium salt of cefotaxime: 95.9%; Water content: 3.8%. EXAMPLE 3 Production of cefotaxime. 228.3 g of 7-ACA are suspended in 720 ml of acetone.

Cefotaxime, 1gram/vial, SDV, 10mL, 25 Vials/Tray McGuff Medical Products
Abstract. Cefotaxime is a new 'third generation' semisynthetic cephalosporin administered intravenously or intramuscularly. It has a broad spectrum of activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria, and is generally more active against Gram-negative bacteria than the 'first' and 'second generation' cephalosporins.
Cefotaxime SITIMIXIE Sahar Pharma
U.S. Patent No. 4,767,852 discloses a process for the preparation of known 2-oxyiminoacetamido-3-cephem-4-carboxylic acid derivatives, including cefotaxime and ceftriaxone, by acylating 7-amino-3-cephem-4-carboxylic acid derivatives already substituted at the 3-position with 2-mercaptobenzothiazolyl- (Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetate of formula (III), the latter being often.

CEFOTAXIME 1g Powder for Injection Bernofarm
A population pharmacokinetic model was developed to describe cefotaxime pharmacokinetics and associated variability in adult ICU patients. The estimated creatinine clearance partly explained the IIV in cefotaxime clearance. However, the remaining unexplained IIV is high and suggests a need for dose individualisation using therapeutic drug.